
Research
Takatori Lab combines theory and experiment to understand and control nonlinear dynamical systems.

Imaging and data-driven discovery of constitutive relations for complex fluids
We discover nonlinear constitutive relations of complex fluids with unknown dynamics. Our goal: Give us any fluid and we'll tell you its equation of motion by using quantitative microscopy and data-driven models constrained by domain knowledge in transport phenomena. We aim to study chemically-reactive fluids with coupling to mass/heat transport.
No, we have not gone off to the dark side. Data science is just a tool, not the focus, for our study. Deep knowledge in soft matter and fluid mechanics is essential for using tools effectively and robustly in transport phenomena.

Interactive simulator of particles in Maxwell viscoelastic fluid
Generated by ChatGPT GPT-5 with prompt:
"Create a Stokesian dynamics simulator in html, where I have one sphere held in the center, and another outer sphere that translates in a circular motion around the center sphere. There is a far-field hydrodynamic force that will cause the center sphere to rotate. Both particles are torque free. The surrounding fluid is a non-Newtonian Maxwell fluid. I want to vary the distance between the two spheres, and also the translational velocity of the outer sphere. Color-code the spheres with a half-coating so that we can easily visualize the sphere rotations."
... with some key modifications, including fixing the hydrodynamic function (i.e., a major technical detail). Still need to know physics in the era of AI!
Try clicking on "Stop motion" button and seeing angular velocity response of center colloid.
Forecasting and controlling dynamical systems
We forecast and control the behavior of complex systems and materials, many with unknown or partially-known dynamics. We perform particle-based simulations and experiments, and use the data to discover "gray-box" models of dynamical systems, including many-body interactions (e.g., interparticle and hydrodynamic fluid interactions).
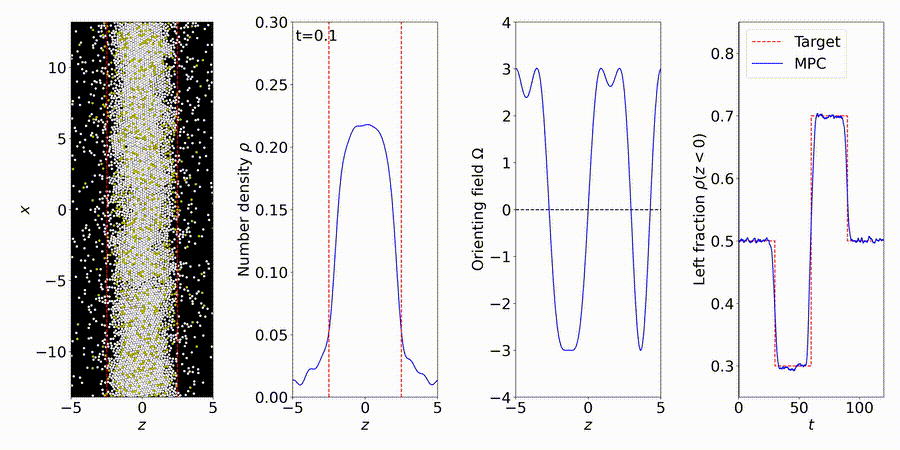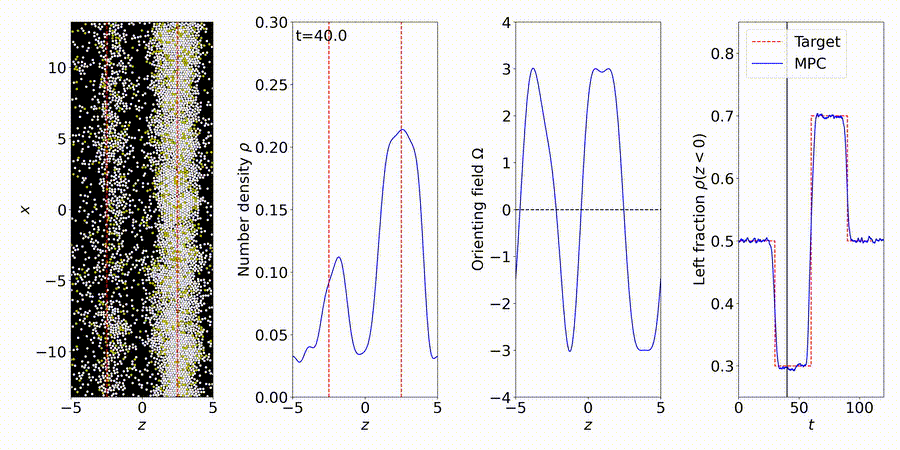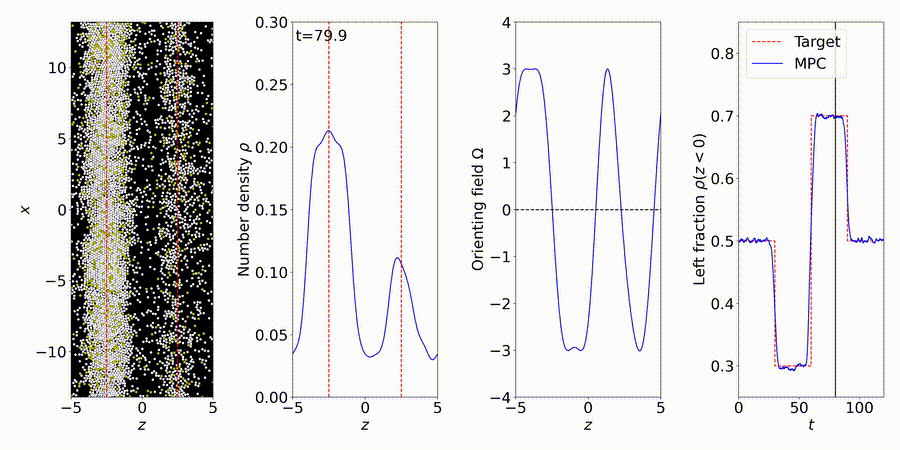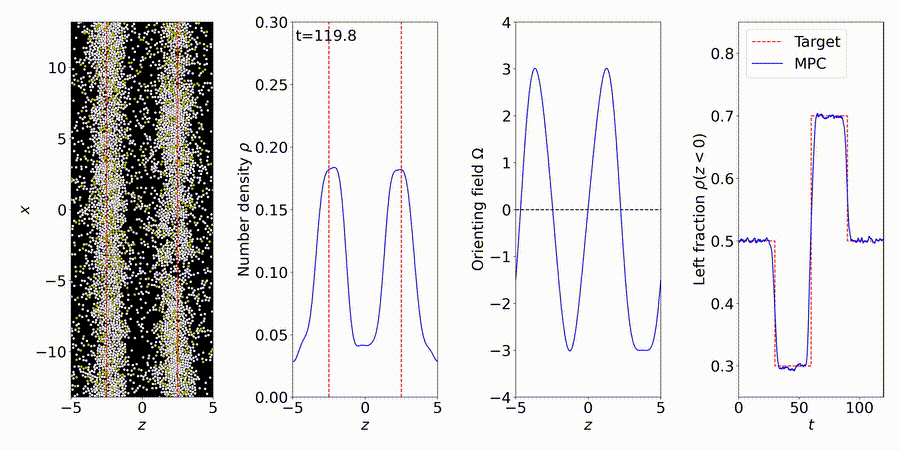
More details: https://titusswsquah.github.io/graybox_abp_mpc/

Past work
Dynamics of soft materials with active forcing
Soft, flexible materials often interact with self-driven microscopic objects. Our lab studied the interplay between fluid interfaces and mechanical forces.
1. Phase separation of 2D lipid droplets. Concepts of phase separation are well-established for materials at equilibrium. What happens when you add chaotic active mixing into this picture?
Arnold DP*, Gubbala A*, Takatori SC. Active surface flows accelerate the coarsening of lipid membrane domains. PRL, 2023. (* equal contribution) [Link] [video abstract]
Gubbala A*, Arnold DP*, Jena A, Anujarerat S, Takatori SC. Dynamic swarms regulate the morphology and distribution of soft membrane domains, PRE, 2024. (* equal contribution) [Link] [video abstract]
Arnold, Takatori. Lipid membrane domains control actin network viscoelasticity. [Link]
2. Membrane vesicles. Here's what happens when we add motile bacteria inside of an enclosure with soft, flexible membranes. The statistics of the membrane edges jiggling around is quite different from equilibrium thermal fluctuations.
Takatori*, Sahu A*. Active contact forces drive non-equilibrium fluctuations in membrane vesicles. PRL. 2020. (* equal and co-corresponding authors) [Link] [video abstract]
3. Active matter in complex environments. Motile agents like bacteria can team up to generate collective swarming flows. The way they move collectively is very different from solitary agents suspended in a fluid.
3-min overview of research area:
Modica, Takatori. Soft confinement of self-propelled rods: simulation and theory. Soft Matter, 2024. [Link] [video abstract]Modica, Omar, Takatori. Boundary design regulates the diffusion of active matter in heterogeneous environments. Soft Matter, 2023. [Link] [video abstract]
Barakat*, Modica*, Lu, Anujarerat, Choi, Takatori. Surface topography induces and orients nematic swarms of active filaments: considerations for lab-on-a-chip devices, ACS Appl Nano Mat, 2024. (* equal contribution) [Link]
Modica, Xi, Takatori. Porous media micro-structure determines the diffusion of active matter: experiments and simulations. Front. Physics. 2022. [Link] [video abstract]
Takatori, Mandadapu. Motility-induced buckling and glassy dynamics regulate three-dimensional transitions of bacterial monolayers. 2020. [Link] [video abstract]
Engineering a dynamic bond
By functionalizing colloidal particles with surface-mobile species, we engineered macromolecular surfactants with tunable colloidal interactions and assembly.


Xu, Jandhyala, Takatori. Dynamic surfactants drive anisotropic colloidal assembly. JCP, 2024. [Link]
Xu, Takatori. Nonequilibrium interactions between multi-scale colloids regulate the suspension microstructure and rheology. Soft Matter, 2023. [Link] [video abstract]
Xu*, Choi*, Nagella, Takatori. Dynamic interfaces for contact-time control of colloidal interactions. Soft Matter, 2023. (* equal contribution) [Link] [video abstract]
Cell surface biophysics
We developed macromolecular probes to quantify the surface crowding of living mammalian cells.

Arnold, Xu, Takatori. Antibody binding reports spatial heterogeneities in cell membrane organization. Nature Commun, 2023. [Link]
Takatori*^, Son*^, Lee, Fletcher^. Engineered molecular sensors for quantifying cell surface crowding. PNAS, 2023. (* equal contribution; ^ co-corresponding authors) [Link]
Son, Takatori, Belardi, Podolski, Bakalar, Fletcher. Molecular height measurement by cell surface optical profilometry (CSOP). PNAS. 2020. [Link]
Very past work: Swim Pressure of Active Matter
Seminar to the general scientific audience.
Our current work has taken quite a random walk from this initial work:
